Abstract
Background: Pyruvate kinase (PK) deficiency is a rare hereditary hemolytic anemia caused by mutations in the PKLR gene encoding the red blood cell (RBC) PK enzyme (PKR). Defects in PKR lead to chronic hemolysis and anemia, which are associated with serious complications, regardless of transfusion status.
Mitapivat (AG-348) is a first-in-class, oral, allosteric activator of PKR that is approved by the US Food and Drug Administration for the treatment of hemolytic anemia in adults with PK deficiency. Mitapivat demonstrated significant improvements in hemoglobin (Hb) in patients (pts) who were not regularly transfused (ACTIVATE, NCT03548220) and significant reduction in transfusion burden in pts who were regularly transfused (ACTIVATE-T, NCT03559699) with PK deficiency.
Methods: Data are reported as of 27Mar2022 of the long-term extension (LTE) study (NCT03853798) of ACTIVATE and ACTIVATE-T. In ACTIVATE, 80 pts (≥18 years [yrs]) with PK deficiency who were not regularly transfused (≤4 transfusion episodes in the prior yr; none in the prior 3 months [mos]) were randomized 1:1 to receive mitapivat or placebo (PBO). Primary endpoint was Hb response (≥1.5 g/dL increase in Hb from baseline [BL] sustained at ≥2 scheduled assessments at weeks [wks] 16, 20, and 24 in the fixed-dose period).
In ACTIVATE-T, 27 pts (≥18 yrs) with PK deficiency who were regularly transfused (≥6 transfusion episodes in the prior yr) were treated with mitapivat. Primary endpoint was transfusion reduction response (≥33% reduction in number of RBC units transfused during the fixed-dose period, compared with the pt's individual transfusion burden history standardized to 24 wks). Transfusion-free status (no transfusions in fixed-dose period) was a secondary endpoint. Further details on study design for each trial have been reported previously.
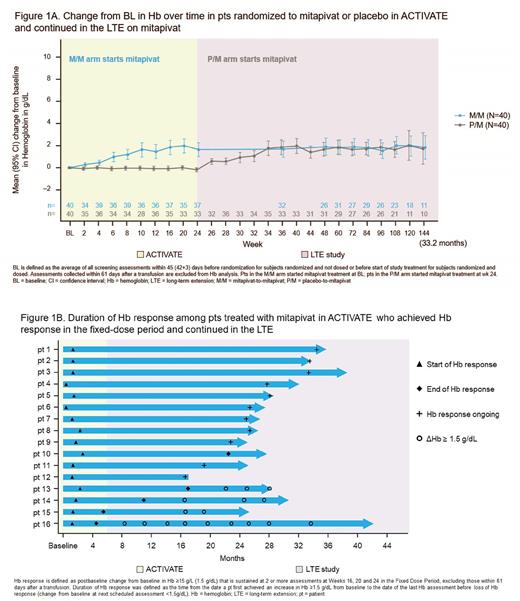
Pts who completed the fixed-dose period of ACTIVATE or ACTIVATE-T were eligible to continue in the LTE, where all pts received mitapivat. In ACTIVATE/LTE analysis, data are reported on pts assigned to mitapivat who achieved Hb response and continued to LTE (mitapivat-to-mitapivat arm [M/M]), and pts originally assigned to placebo who switched to mitapivat in LTE (PBO-to-mitapivat arm [P/M]) and then met Hb response criteria. Duration of Hb response in M/M pts was defined as time from date a pt first achieved an increase in Hb ≥1.5 g/dL from BL to the date of last Hb assessment with an increase in Hb ≥1.5 g/dL from BL before loss of Hb response (change from BL <1.5g/dL). ACTIVATE-T/LTE analysis assessed transfusion reduction response in the ACTIVATE-T and LTE, and transfusion-free duration among pts who achieved transfusion-free status in ACTIVATE-T.
Results: In ACTIVATE, 40% (16 out of 40) of pts treated with mitapivat achieved a Hb response at 24 wks; in the LTE, 39.5% (15 out of 38) of pts randomized to PBO in ACTIVATE showed a Hb response at 24 wks after switching to mitapivat. These improvements were sustained with continued treatment up to 33.2 mos (Figure 1A). Improvements in markers of hemolysis and hematopoiesis were also sustained. The median duration of Hb response among the 16 ACTIVATE Hb responders was 21.9 mos with responses ongoing up to 32.9 mos (Figure 1B). Five pts fell below the 1.5g/dL response threshold; however, 4 of the 5 returned to a change from BL ≥1.5 g/dL at multiple timepoints afterwards, showing continued benefit from mitapivat treatment (Figure 1B).
In ACTIVATE-T, 37% (10 of 27) of pts achieved a transfusion reduction response and 22% (6 pts) achieved transfusion-free status. In the ACTIVATE-T/LTE, 37% (10 of 27) of pts met criteria for a transfusion reduction response up to 38.3 mos of fixed-dose period. All 6 pts who achieved transfusion-free status in ACTIVATE-T maintained the status in the LTE up to 38.3 mos. The median duration of transfusion-free response was 33.4 mos.
Mitapivat has been well tolerated, with extended treatment duration in the LTE studies showing no new safety findings and consistent with previous studies.
Conclusions: In pts with PK deficiency, treatment with mitapivat continues to demonstrate long-term and durable improvements in Hb and reduction in transfusion burden over several years. Combined with a favorable safety profile, these data continue to support the long-term use of mitapivat as the first disease-modifying drug therapy approved for adults with PK deficiency and its clear potential for real-word benefits in these pts.
Disclosures
Grace:Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy; Novartis: Research Funding; Agios Pharmaceuticals: Consultancy, Research Funding. Glenthøj:Saniona: Research Funding; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees; bluebird bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Pharmacosmos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding. Barcellini:Incyte: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Speakers Bureau; Momenta: Honoraria; Apellis: Honoraria; Janssen: Honoraria; Agios: Honoraria, Research Funding; Novartis: Honoraria; Biocryst: Honoraria; Alexion: Honoraria; SOBI: Honoraria; Bioverativ: Membership on an entity's Board of Directors or advisory committees. Verhovsek:Vertex: Consultancy. Rothman:Pfizer: Consultancy, Honoraria, Research Funding; Agios: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; bluebird bio: Research Funding. Morado:Sanofi Genzyme: Honoraria, Other: Grants. Layton:Cerus: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Galactéros:Addmedica: Membership on an entity's Board of Directors or advisory committees. van Beers:Agios Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Pfizer: Research Funding; RR Mechatronics: Research Funding; Sobi: Research Funding; Sanofi: Consultancy; Global Blood Therapeutics: Consultancy. Viprakasit:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Agios Pharmaceuticals: Consultancy, Research Funding; Ionis Pharmaceuticals: Consultancy, Research Funding; La Jolla Pharmaceuticals: Consultancy, Research Funding; Protagonist Therapeutics: Consultancy, Research Funding; Vifor Pharma: Consultancy, Research Funding. Chonat:Daiichi Sankyo: Consultancy; Forma Therapeutics: Consultancy; Novartis: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy, Research Funding; Alexion: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; Agios: Consultancy, Research Funding. Judge:Agios: Current Employment, Other: Stockholder. Kosinski:Agios: Consultancy, Other: Shareholder. Hawkins:Agios: Current Employment, Other: Stockholder. Gheuens:Agios: Current Employment, Other: Stockholder. Xu:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company. McGee:Agios Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Beynon:Agios: Current Employment, Other: Stockholder. Al-Samkari:Agios: Consultancy, Research Funding; Sobi: Consultancy, Research Funding; Amgen: Research Funding; Novartis: Consultancy; Rigel: Consultancy; argenx: Consultancy; Forma: Consultancy; Moderna: Consultancy; Dova: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal